In the non-cubic chalcopyrite structural compounds, a "pseudo-cubic" structure design has been developed to achieve a highly degenerate energy band similar to that of cubic structures. This approach enhances both electrical and thermoelectric performance. (a) A schematic of the cubic sphalerite structure and its energy band diagram is shown. (b) The crystal and energy band structure of a ternary chalcopyrite compound are illustrated. The "cubic" structure features a cubic cation framework combined with a non-cubic anionic lattice. (c) The relationship between the ZT value and the valence band top splitting ΔCF is presented. Red dots represent high-temperature compounds with ZT > 1, while blue and green dots correspond to (Ag,Cu)InTe2 and Cu(In,Ga)Te2 solid solutions, respectively.
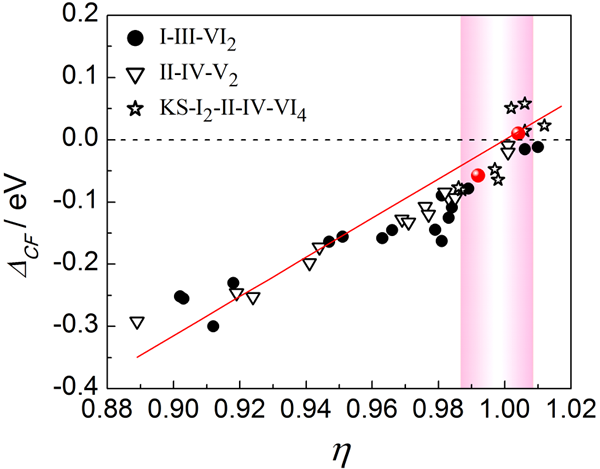
Figure 2 introduces the Unity-η screening criterion. The red dots represent high-temperature compounds with ZT > 1, while the pink band marks the region where η ≈ 1—potentially indicating areas where new thermoelectric materials might be found.
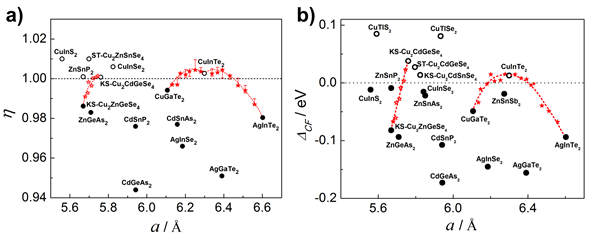
Figure 3 illustrates the design of η vs. a and ΔCF vs. a for solid solution compounds. The red star highlights two compounds with η < 1 (ΔCF < 0) and η > 1 (ΔCF > 0). The red dotted line shows the overall trend.
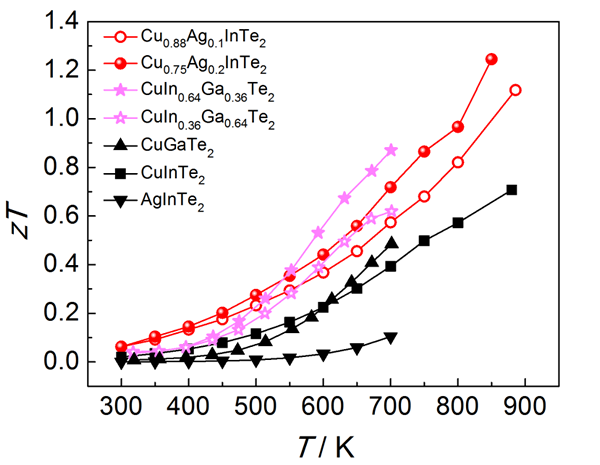
Figure 4 presents the thermoelectric performance of CuInTe2, AgInTe2 (CuGaTe2), and their solid solutions.
Developing industrial applications for high-performance thermoelectric materials is a major goal in thermoelectric science and technology. The performance of these materials is typically evaluated using the dimensionless figure of merit, ZT, which depends on the Seebeck coefficient, electrical conductivity, thermal conductivity, and absolute temperature. Historically, research has focused on materials like SiGe, PbTe, skutterudite, Half-Heusler, Mgâ‚‚Si, and Cuâ‚‚Se, all of which have highly symmetric cubic structures. These structures often lead to highly degenerate or multi-valley energy bands, resulting in good electrical transport properties.
However, many natural compounds have non-cubic structures, featuring narrow bandgaps and low thermal conductivity. Despite this, they have largely been overlooked by researchers. Identifying new thermoelectric materials from these non-cubic systems remains a significant challenge.
Diamond-like compounds, derived from the diamond structure, consist of tetrahedral units with SP3 hybrid bonds. Differences in atomic radii and chemical valences cause lattice distortion, leading to reduced thermal conductivity. By altering the elemental composition, the bandgap can be tuned across a wide range, from narrow-band semiconductors (under 1 eV) to wide-bandgap semiconductors or insulators. This combination of low thermal conductivity and adjustable electrical properties makes them promising candidates for thermoelectric applications.
Earlier studies by the Shanghai Institute of Ceramics showed that CuInTe2, a tetragonal quasi-diamond structure, achieved a ZT of 1.18 at 850 K. Later, Japanese researchers reported CuGaTe2 with a ZT of up to 1.4, demonstrating that diamond-like materials can rival traditional thermoelectrics. However, the mechanism behind their high performance remained unclear, limiting further exploration.
Recently, researchers from the Shanghai Institute of Ceramics, along with collaborators from the University of Washington and the University of Michigan, used non-cubic chalcopyrite compounds as a model system. Through theoretical calculations and experiments, they proposed the "pseudo-cubic" microstructure design strategy. This approach involves forming a long-range ordered cubic-like cation framework while allowing anion lattices to remain distorted, thereby optimizing both electrical and thermal transport.
The study identified the Unity-η rule, where when the square parameter η = c/2a is close to 1, the valence band splitting ΔCF approaches zero. This leads to optimal electrical performance and high ZT values. High-performing materials like CuInTe2 and CuGaTe2 align with this rule.
Using the Unity-η screening method, potential thermoelectric compounds can be efficiently identified among multiple chalcopyrite structures. For materials where η is not close to 1, a solid solution design principle was introduced. By combining two or more compounds with small lattice mismatches, adjusting the composition can bring η closer to 1, improving thermoelectric performance.
Three solid solutions were designed based on the η(ΔCF) vs. a map, and experimental verification showed significant improvements in ZT compared to the base materials. The "pseudo-cubic" microstructure concept offers a powerful new approach for discovering and designing high-performance non-cubic thermoelectric materials.
Kitchen Islands & Carts,Kitchen Shelf Racks,Food Hand Carts,Storage Carts
Changzhou Offistyle Furniture Co., Ltd. , https://www.offistylefurniture.com